Overview of the Montelukast Intermediate Market
The Montelukast intermediate market is witnessing steady growth globally, driven primarily by the rising prevalence of respiratory diseases such as asthma and allergic rhinitis. Montelukast, a leukotriene receptor antagonist, is a critical drug used to manage asthma symptoms by blocking substances that cause airway inflammation and bronchoconstriction. The synthesis of Montelukast requires complex chemical intermediates, making the intermediate market vital for pharmaceutical manufacturers.
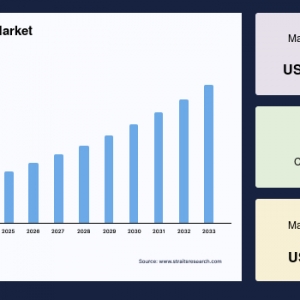
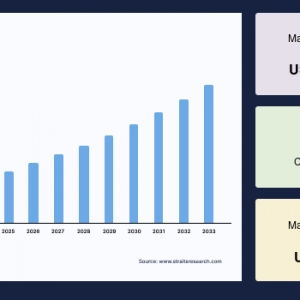
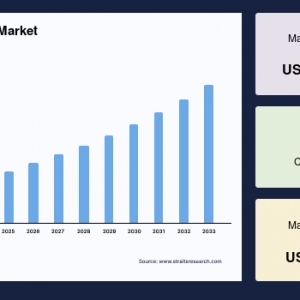
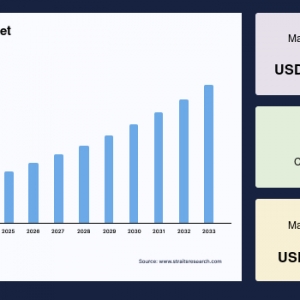
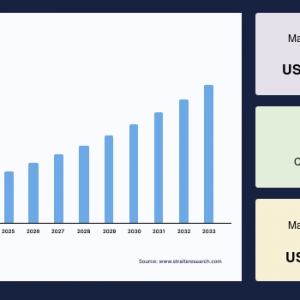
The global montelukast intermediate market was valued at USD 7.57 million in 2024 and is projected to reach from USD 7.93 million in 2025 to USD 11.46 million by 2033, growing at a CAGR of 4.71% during the forecast period (2025-2033).
Key Market Drivers
The primary driver of the Montelukast intermediate market is the growing global prevalence of asthma. Environmental factors such as urbanization, increased air pollution, and lifestyle changes have contributed to a surge in asthma cases, particularly in densely populated regions. For example, in India, studies estimate that approximately 2.05% of the population over 15 years is affected by asthma, accounting for around 18 million individuals. Globally, asthma affects more females than males, with 10.8% of adult females suffering from the condition compared to 6.5% of adult males. It is also the most common chronic illness in children, with millions affected worldwide.
The increased awareness of asthma and allergy management options has resulted in higher demand for Montelukast as a treatment, fueling the need for its pharmaceutical intermediates. Additionally, ongoing research and innovation efforts to develop sustained-release formulations and advanced drug delivery systems, such as nanoparticle-based techniques, are expected to further boost market growth. These advancements offer benefits like extended therapeutic effects and improved patient adherence to treatment regimens.
Market Restraints and Challenges
Despite robust demand, the Montelukast intermediate market faces challenges, primarily from stringent regulatory requirements. Manufacturing pharmaceutical intermediates involves adherence to Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and comprehensive quality controls. Regulatory bodies such as the U.S. FDA and European Medicines Agency impose strict guidelines that intermediates producers must follow to ensure safety, efficacy, and product quality. Failure to comply can lead to delays in product approvals, market entry restrictions, or legal consequences.
Moreover, the complex and multi-step chemical synthesis process involved in producing Montelukast intermediates demands advanced expertise and investment in technology, which may pose barriers for new entrants. Fluctuations in raw material prices and supply chain disruptions also add uncertainty to production costs and timelines.
Technological Innovations and Product Developments
The Montelukast intermediate market is evolving with significant technological advancements aimed at improving synthesis efficiency and product quality. Innovation focuses on optimizing synthesis routes to reduce costs and environmental impact, such as the adoption of green chemistry principles and process automation.
Manufacturers are also exploring advanced pharmaceutical formulations. The development of sustained-release Montelukast products aims to overcome the limitations of immediate-release tablets, which require multiple daily doses. Technologies like microencapsulation and nanoparticle-based drug delivery can enable controlled release of the active drug, improving therapeutic outcomes and patient compliance.
Regional Market Insights
Asia-Pacific leads the global Montelukast intermediate market in terms of size and growth potential. Factors such as rapid industrialization, increased air pollution, and expanding healthcare infrastructure are major contributors. Countries like China, India, and South Korea benefit from skilled pharmaceutical workforces and cost-effective manufacturing capabilities, making the region a hub for pharmaceutical intermediate production.
India, in particular, has a significant patient population suffering from respiratory diseases. According to the World Health Organization, the Asia-Pacific region accounts for one-third of all air-pollution-related deaths globally, exacerbating conditions like asthma. The robust pharmaceutical sector in this region, supported by government initiatives and foreign investments, fuels the growth of the Montelukast intermediate market.
Europe is identified as one of the fastest-growing markets due to the presence of large pharmaceutical companies, high healthcare standards, and increasing asthma prevalence. North America remains a significant market owing to advanced healthcare infrastructure and high patient awareness. Meanwhile, emerging markets in Latin America, the Middle East, and Africa show moderate growth potential driven by improving healthcare systems and rising disease awareness.
Market Segmentation
The Montelukast intermediate market is segmented by application into asthma, bronchospasm, allergic rhinitis, and urticaria. Among these, the asthma segment holds the largest share because Montelukast is primarily prescribed to treat asthma symptoms by reducing airway inflammation and bronchoconstriction.
From an end-user perspective, the pharmaceutical and biotechnology sectors dominate the market. These companies are heavily involved in research, development, and manufacturing of Montelukast and its intermediates. They invest substantially in optimizing synthesis processes and ensuring compliance with regulatory standards to maintain safety and efficacy. Contract manufacturing organizations (CMOs) and research institutes also form a part of the ecosystem, supporting scale-up production and innovation.
Industry Outlook
The global Montelukast intermediate market is expected to maintain steady growth over the forecast period. The growing global burden of asthma and allergic diseases will continue to sustain demand for Montelukast as a treatment, consequently driving the need for high-quality intermediates. Innovations in drug formulations and delivery systems will provide additional growth avenues.
However, manufacturers must navigate regulatory challenges by implementing rigorous quality control and compliance measures. Investment in advanced synthesis technologies and sustainable manufacturing practices could provide a competitive edge. Strategic collaborations and regional expansions, particularly in emerging markets, offer opportunities to capture new customer bases and increase market share.